Barker’s Hydrogel Eliminates DOX Side Effects
Fourteen mice scurried around in laboratory cages, eating, sniffing, and running peacefully.
You would never guess that, several weeks earlier, half of them had received five times the lethal dose of a leading chemotherapy drug.
Doxorubicin (DOX) is one of the most effective chemotherapies approved for treatment of solid tumors in the United States. Unfortunately, because DOX and other chemotherapies are administered intravenously, they often accumulate in nontarget tissues and organs—leading to a variety of serious health problems for cancer survivors.
“Childhood cancer survivors are a high-risk group for many severe or life-threatening chronic health conditions, including second cancers, cardiovascular and endocrine diseases, renal dysfunction, and severe musculoskeletal problems,” said Elizabeth Barker, an assistant professor in UT’s Department of Mechanical, Aerospace, and Biomedical Engineering (MABE). “The risk continues to increase years after therapy is complete.”
The St. Jude Lifetime Cohort Study, published by St. Jude Children’s Research Hospital (St. Jude) in 2021, found that 99.9% of children whose tumors are treated with chemotherapy develop chronic health conditions later in life.
“What we need is a way to administer the drugs directly to the tumor site and keep them there,” Barker said.
Barker first started investigating this idea as a master’s student in UT’s Department of Materials Science and Engineering. With instruction from her advisor, the late Roberto Benson, she began developing a novel hydrogel: a hydrophobic polymer that retains its structure after absorbing large amounts of water.
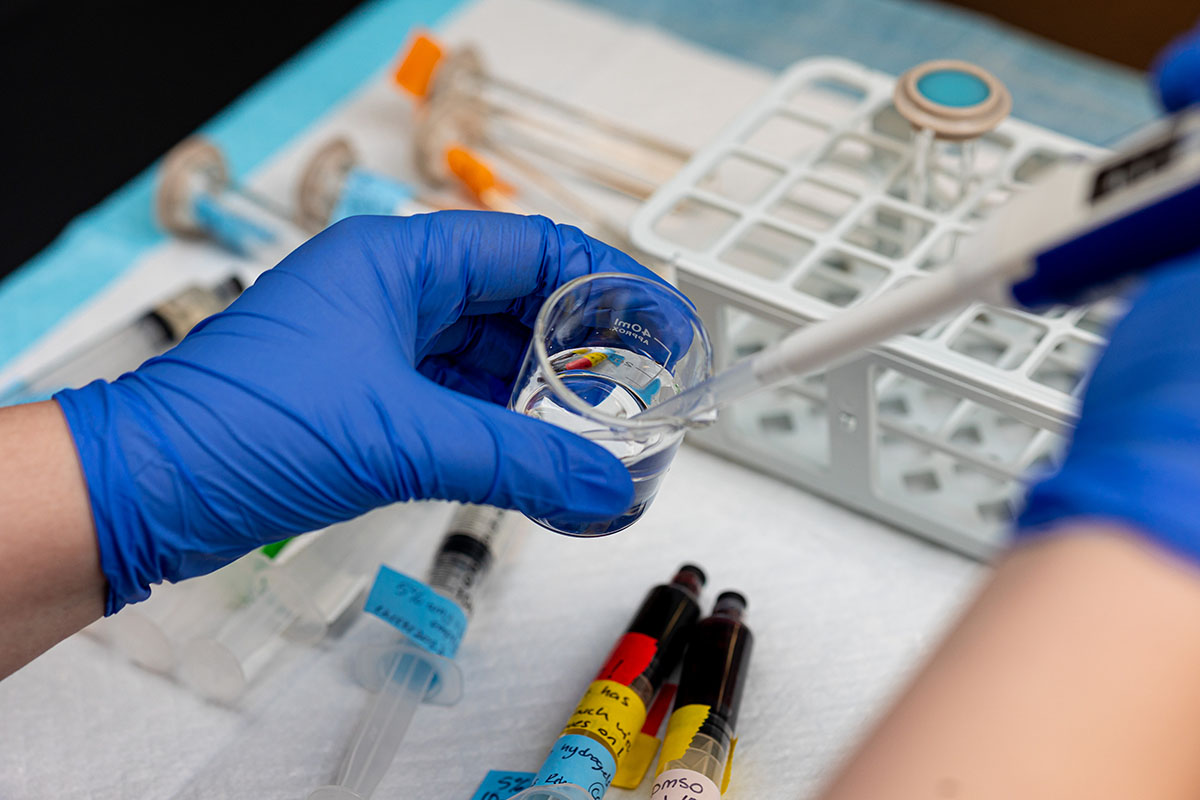
After years of development, the final product is Amygel: an injectable, biodegradable starch hydrogel. Injecting tissues with a mixture of Amygel and a chemotherapy drug like DOX not only improves chemotherapy distribution within the tumor—up to 10 mm from the injection site—but prevents the drug from leaving the tumor.
“Amygel is compatible with many kinds of drug compounds,” Barker said. “It is a platform technology that can be used to deliver treatments for lots of different diseases.”
Barker and her students recently demonstrated the efficacy of the Amygel/DOX delivery system on mice that had been implanted with cells of a human pediatric medulloblastoma, the most frequently occurring malignant brain tumor in pediatric patients.
Half the tumors were injected with a single low, but therapeutic, 10-microliter (μL) dose of DOX. The other half tested the furthest limits of Amygel, receiving a whopping milligram of DOX—about five times the lethal dose for an average mouse.
The results were dramatic. By the end of the six-week trial, not only were all the mice still alive and showing no side effects from the injection, but every one of the Amygel/DOX-treated tumors had shrunk by at least 20%.
In fact, more than half of them had undergone a complete response, with no cancer cells left alive.
Just as significantly, Barker and her team could find no evidence of DOX in the non-target tissues or organs in any of the mice. If that result were repeated in humans, it would eliminate the risk of chemotherapy-derived health complications.
“Amygel let us achieve a complete response to treatment with a single 10-μL injection—a fraction of a drop—and with no systemic side effects,” Barker said.
With these groundbreaking results in hand, Barker approached the director of St. Jude’s Lead Discovery Informatics Center (LDI), Anang Shelat. The LDI is focused on discovering new drugs and developing them into treatments specifically for pediatric cancer patients. Shelat jumped on the chance to test the new drug delivery system.
Last November, Barker personally drove to St. Jude to deliver loaded syringes of Amygel—then drove straight back to Knoxville.
“I want to test the usability of the gel by seeing how easily other researchers can perform the experiments, and whether they achieve the same results I would get if I injected the tumors myself,” she said.
St. Jude’s LDI has concluded its first preclinical experiment with Amygel, which resulted in effective drug delivery with no systemic side effects, and Shelat is planning several more. So far, tests of Amygel at the drug development company Charles River Laboratories are equally promising.
“As an inventor or creator, we know our discoveries work in our hands,” Barker said. “The true test is if someone else can use the invention we’ve created and get the same results we do.”
Contact
Izzie Gall (865-974-7203, egall4@utk.edu)
